
Push Your Life Sciences Business to New Success with a Content Management for Regulated Industries Solution
Is your life science business constantly caught between the competing demands of product innovation and regulatory compliance?
Increased competition, decreasing product pipelines, and the rising costs associated with drug development and commercialization only add to the pressures. Meanwhile, the need to accelerate your time-to-market remains constant. Achieving all of these ambitions, in businesses that are becoming ever more data and document intensive, requires clarity, organization and security.
TerraLink’s content management for regulated industries solution, which is based on OpenText™ Regulated Information Management, provides convenient access to all documents within a controlled environment.
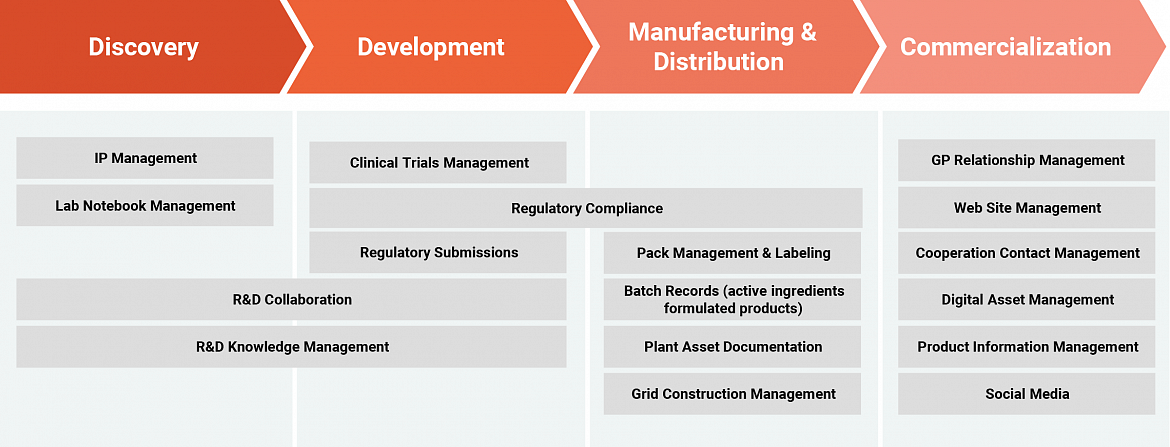
Regulated information management across the life science value chain
It enables disparate teams and departments to access the same shared information at the same time, with users connecting directly via applications such as Microsoft Word or Adobe Acrobat through its desktop integration features. And by operating across all document lifecycle phases, from authoring, review and approval through to release, change, retirement, and archiving, it instantly provides businesses with greater control and enhanced productivity.
Life science businesses, such as those in the pharmaceutical, biotech, and cosmetic sectors, now operate in an increasingly regulated environment. At both federal and international level, regulations are placing ever more complex demands on CIOs who are already battling to ensure their systems are as robust as possible in order to facilitate an accelerated time-to-market.
The need to manage documents in compliance with FDA 21 CFR Part 11 and EU Annex 11 regulations adds to the pressures already facing businesses looking to maintain all controlled documents in an orderly fashion. At the same time, huge quantities of information stored on legacy systems also need to be made available to all stakeholders.
Thankfully, with TerraLink’s solution it is easier than ever to manage and control key documents throughout their lifecycles via features including electronic signatures, approval workflows, metadata management, and version control.
The diagram above shows the seven questions that need to be answered to use hidden knowledge. We should know where to find the information sources, understand the information we gather, and know how to structure and store it. Finally, we need to know how to share and actualize knowledge, and how we can find it in future.
TerraLink’s content management for regulated industries solution provides world-class control for life science companies. By installing a single, highly scalable content repository businesses become instantly capable of effectively managing millions of documents – including text, spreadsheets and drawings.
The nine-level permission model – including controls such as See, Modify, Edit Attributes, Reserve, and Delete – allows you to maintain ultimate security, enabling only appropriate users to conduct actions at a level representing their seniority within the business. Extensive version control features, including change histories and audit trails that reveal when, what and why changes were made to documents, further strengthen your position.
- One client reduced its document approval processing costs by 60% in one year.
- Another saw increased productivity by reducing manual document processing time by 66%.
- And another achieved 100% regulatory compliance using the content management for regulated industries solution.
TerraLink’s content management for regulated industries solution offers integration with leading point solutions, such as eSubmissions, eTMF, QMS, LIMS, LMS, and PV, as well as with ERP and SharePoint. It provides an environment in which life science business can securely create, process and dispose of documents, with role-based viewing and printing reinforcing its inherent security.
Configurable workflows reduce the initial validation effort and remove the need for additional re-validation if and when workflows change. And by enabling businesses to easily comply with regulations and maintain auditability, the threat of warning letters and plant shutdowns is reduced.
TerraLink’s content management for regulated industries solution can also:
- Enhance competitive advantage and accelerate time to market by providing an efficient system in which documents are managed and automatically comply with FDA 21 CFR Part 11 and EU Annex 11.
- Increase productivity and efficiency by providing a single repository for securely organizing, controlling, and sharing enterprise content.
- Minimize change management and training costs by providing access to regulated content via familiar desktop applications.
- Create business value and achieve strategic success with an intuitive workflow designed to provide security, control and appropriate accessibility for all content throughout its entire lifecycle.
Why Choose TerraLink for Your Content Management for Regulated Industries Solution?
For more than 25 years, we have helped organizations around the world implement enterprise IT solutions focused on stability, scalability and innovation. We’ve completed more than 150 major ECM projects in seven countries across three continents, giving us an international perspective and enabling us to quickly determine the optimal solution to fit your circumstances.
More than 200,000 global users benefit from TerraLink ECM solutions
A team of 200+ skilled professionals are ready to implement your solution
The average TerraLink team member has 11.4 years’ experience
TerraLink has been an OpenText™ Platinum partner since 2009
Our portfolio includes both cloud and on-premise implementations
Guaranteed stability, scalability and innovation
- Contract and Legal Case Management
- Invoice Processing
- Engineering Document Management
- Extended Enterprise Content Management
- Employee Document Management
- Knowledge Management
- Content Management for Regulated Industries
- Document Capture and Automation
- OpenText Extended ECM for Oracle E-Business Suite
- TerraLink ECM Mobile
Contact us to discuss your requirements

Don't miss a thing... follow us now!
Ready to unlock the information advantage? Keen to know more about becoming an intelligent enterprise? Get every TerraLink update direct through our social networks and advance your digital transformation.